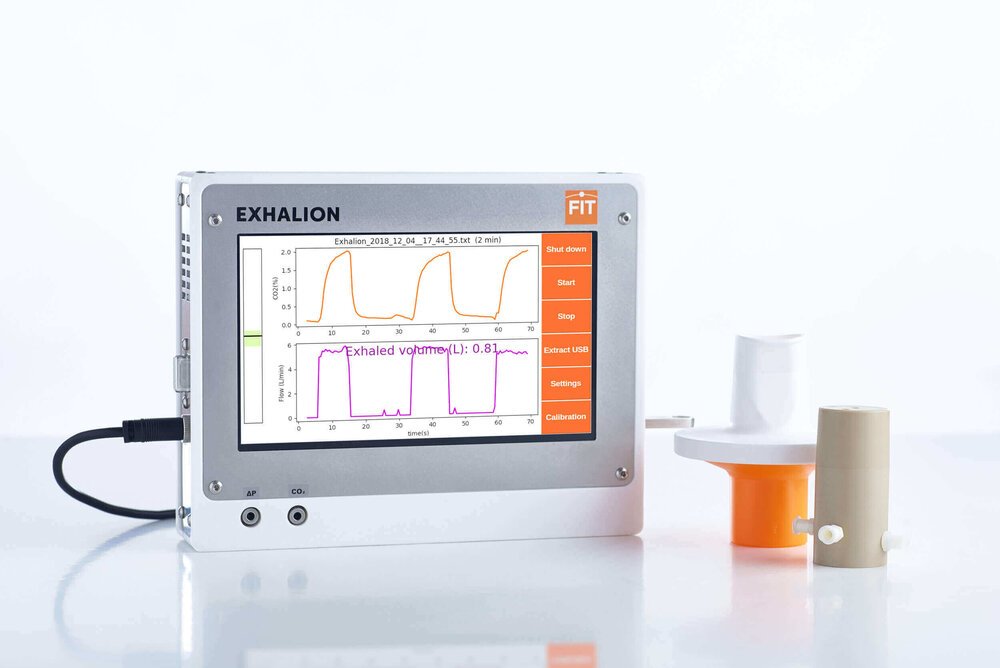
Why SESI is ideal for ionizing low volatility molecules:
- Because ionizing at atmospheric pressure improves limits of detection:
Turbulent losses and condensation losses are eliminated, which means more molecules reach the ionization area. Since molecules are ionized before they reach the adiabatic expansion and cooling, region process (instead of at the entrance of the ionizer as it happens in low-pressure operating systems), ions are heated by electric fields and focused.
The ionization reaction is much faster, which means better ionization efficiency. The velocity scales with (1) the concentration of the vapors and (2) the concentration of the charging ions, both of them go with the pressure in the ionizer. At room pressure (10^3 mBar), the velocity of the charge reaction is 10^6 times higher than at 1 mBar.
High performance Electrospray - MS systems are optimized to transfer and desolvate heavy ions from atmospheric pressure into their vacuum side. This challenging technical problem is already solved by currently available commercial MS systems. SSX just rides very powerful horses!
The ionization efficiency of nano-electrospray is extremely high because the concentration of charging agents near the nano-jet is extremely high
- Because the nano-electrospray provides very clean spectra:
in-source ion fragmentation and oxidation is very low because there are no high energy ions present at any point in the ionizer. Compared with other sources using plasma as a source of charging ions, SESI ions are formed from evaporating nano-droplets. The result is a lot simpler spectra, which are easier to interpret. This is particularly important for untargeted studies, like biomarker discovery.